Scient Spotlight - Swim-mune System
Swim-mune System: The Zebrafish Axillary Lymphoid Organ as a Model for Immune Function
By Dr. Curtis Boswell
The vertebrate immune system is built around a network of organs scattered throughout the body. These organs – termed lymphatic organs – constantly surveil the fluids of the body for nasty intruders like pathogens and viruses. When intruders are detected, these lymphatic organs are the central hubs and highways for immune cells to traffic through the body and fight off the invaders. In mammals, lymph nodes are key lymphatic organs that have roles in housing immune cells so that they’re primed to action when a foreign invader is detected.
Zebrafish were previously thought to lack structures like the mammalian lymph node. Although they have diffuse sites where immune cells cluster and carry out their roles in fighting off pathogens – such as within the kidney and the spleen – the clear functional analog of a lymph node had not been identified. Moreover, these diffuse immune sites are wedged deep within the juvenile and adult zebrafish tissues, making them a real challenge to access for live imaging. This raised a major question: do zebrafish possess a structure that is analogous in form and function to a mammalian lymph node?
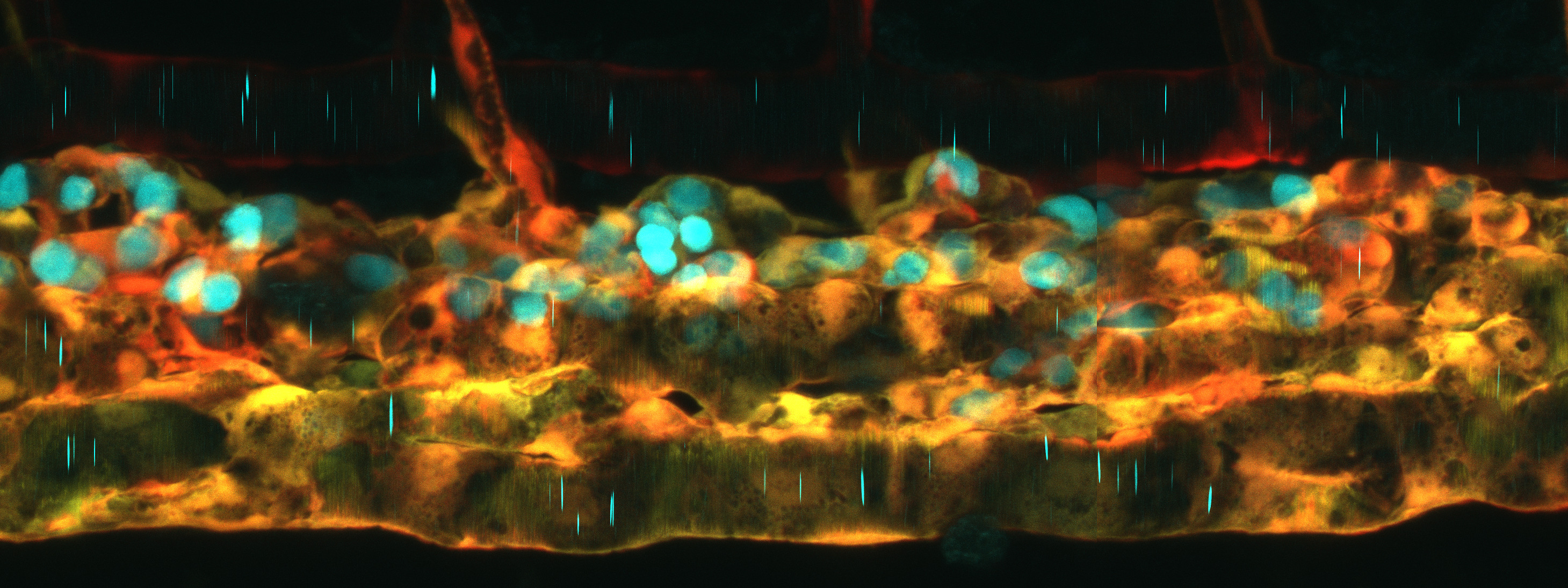
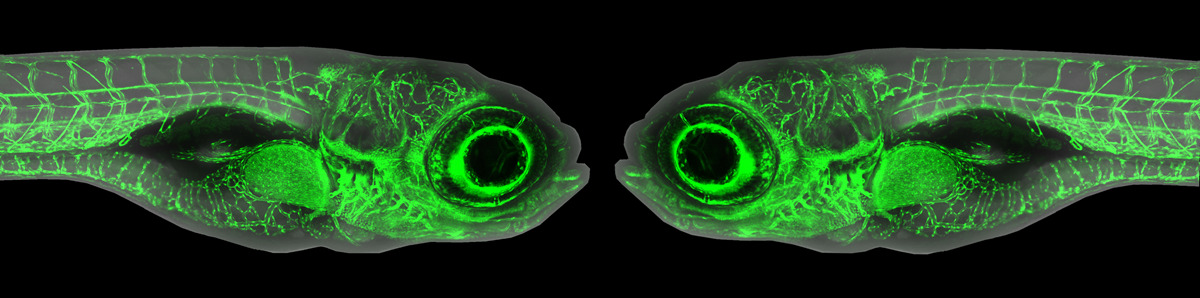
While studying the development of the lymphatic system, Castranova et al. made a surprising discovery. Tucked under the ‘armpit’ of the zebrafish near the base of the pectoral fin, they identified a small lobular translucent tissue. This lobe is packed with lymphatic vessels – a key hallmarks for a lymphatic organ. The authors named this structure the Axillary Lymphoid Organ, or ALO. Excitingly, this organ is just hanging out on the surface of the fish which gave the researchers unparalleled access for both longitudinal and live imaging as well as dissection.
The authors used histological and ultrastructural analyses and found that the ALO is divided into two primary regions, an outer cortical layer, where large populations of immune cells are concentrated, and an inner medullary region, which contains a network of fibroblasts tangled within lymphatic vasculature. From this analysis alone, the ALO looks shockingly similar to mammalian lymph nodes.
Next, the authors plucked some ALOs from the fish and used single-cell RNA sequencing to define the cellular populations they contain. They found the ALO to be a bustling hub of immune cell populations, including antigen-presenting macrophages, naive and activated B cells, and T cells, all of which are critical players in fighting off infections and pathogens. Importantly, the fibroblast cells within the ALO express specialized molecules called chemokines that direct cell migration. Lymphocytes sniff out these molecules to move along defined migratory paths and can enter and exit the ALO when these molecules turn on or off in response to an immune challenge. To support this, the authors used live imaging and saw these immune cells moving in and out of the lymphatic vessels and the ALO cortex, similar to how immune cells navigate mammalian lymph nodes.
The researchers discovered that the ALO is plugged into to an adjacent lymphatic network that drains into a large lymph sac, facilitating immune cell movement and fluid exchange. They injected fluorescent tracers into the body of the fish and watched the molecules get shuttled into the ALO, where they were rapidly processed and broken down. This makes the ALO a highly accessible tissue for studying immune cell-pathogen interactions.
One remarkable feature of the ALO is its ability to capitalize on the regenerative powers of zebrafish. When surgically removed, the ALO was shown to completely regrow within two weeks. This regrowth makes it a powerful model for studying how immune organs develop, respond to threats, and regenerate over time. More importantly, this regenerative ability may open doors for the development of therapies to promote lymph node repair and regeneration in humans, which could be used in the treatment of the wide variety of lymphatic disorders that affect the population.
Finally, the authors dove even deeper into the function of the ALO with live imaging of this newly discovered lymph organ. A common marker for cancer progression in humans is when tumour cells have infiltrated lymph nodes – a prognosis that often indicates substantial cancer growth. Human cancers break away from their site of origin and use the natural highways of the body to move and invade other locations, in a process known as metastasis. Using a zebrafish model of leukemia (known as T-cell acute lymphoblastic leukemia), the authors found that these cancer cells can also infiltrate and accumulate within the ALO. This exciting discovery suggests that the ALO could be an in vivo goldmine for studying how cancers evade immune surveillance, hide out in lymphatic organs and progress beyond their site of origin.
How has the ALO gone unnoticed for so many years? In fact, this fleshy lobe has been used as a taxonomic identifier for cypriniform fishes, but its function had not been investigated. Indeed, without all the powerful transgenic and sequencing-based tools at the disposal of a zebrafish researcher, this lobe would otherwise look like a nondescript blob. On a field trip to the Smithsonian National Museum of Natural History fish collection, the authors examined a small sample of different species of “basal” teleost fish and found ALO-like structures in all fish examined. Zebrafish have once again shed light on an important feature of fish biology that has been previously overlooked!
The discovery of the ALO as a functional immune hub in zebrafish raises several new questions. First, what is the evolutionary significance of this organ? Does it represent a simplified lymph node? Second, how does the ALO coordinate immune surveillance with other diffuse lymphoid tissues in zebrafish, such as the lymphoid tissues found in the kidney or spleen? Additionally, given its accessibility, can the ALO be exploited for in vivo studies of immune interactions with pathogens or therapeutic interventions like drug screens that prevent lymph node metastasis? The answers to these and other questions could position zebrafish as an even more powerful model for immunology, with implications spanning infection biology, cancer research, and immune system evolution.
References
Castranova, D., Kenton, M.I., Kraus, A., Dell, C.W., Park, J.S., Galanternik, M.V., Park, G., Lumbantobing, D.N., Dye, L., Marvel, M., et al. (2024). The axillary lymphoid organ - an external, experimentally accessible immune organ in the zebrafish. bioRxiv, 2024.07.25.605139. 10.1101/2024.07.25.605139.
About the Science Spotlight Writer
 Curtis Boswell is a Postdoctoral Fellow in the Giraldez lab at Yale University. Previously, he completed his Ph.D. in the Ciruna lab at the Hospital for Sick Children (Toronto, Canada) developing and studying models of scoliosis in zebrafish. He is interested in roles of transcription factors in reading the regulatory grammar of the genome and establishing cell identities during early zebrafish development
Curtis Boswell is a Postdoctoral Fellow in the Giraldez lab at Yale University. Previously, he completed his Ph.D. in the Ciruna lab at the Hospital for Sick Children (Toronto, Canada) developing and studying models of scoliosis in zebrafish. He is interested in roles of transcription factors in reading the regulatory grammar of the genome and establishing cell identities during early zebrafish development
 Dan Castranova is an aquatic research specialist in the Weinstein lab at the NIH. In the Weinstein Lab, he studies the development and function of the lymphatic and vascular systems in zebrafish. He co-discovered the intracranial meningeal lymphatic system in zebrafish and contributed to identifying the axillary lymphoid organ (ALO), advancing our understanding of immune surveillance and cerebrovascular health.
Dan Castranova is an aquatic research specialist in the Weinstein lab at the NIH. In the Weinstein Lab, he studies the development and function of the lymphatic and vascular systems in zebrafish. He co-discovered the intracranial meningeal lymphatic system in zebrafish and contributed to identifying the axillary lymphoid organ (ALO), advancing our understanding of immune surveillance and cerebrovascular health.
 Brant Weinstein is the Senior Investigator of the Section on Vertebrate Organogenesis at the NIH. Dr. Weinstein's research focuses on the development of the vascular system, using zebrafish as a model to uncover genetic and molecular mechanisms governing blood and lymphatic vessel formation. His lab has pioneered imaging techniques and genetic tools to study endothelial cell behavior and vascular patterning in vivo.
Brant Weinstein is the Senior Investigator of the Section on Vertebrate Organogenesis at the NIH. Dr. Weinstein's research focuses on the development of the vascular system, using zebrafish as a model to uncover genetic and molecular mechanisms governing blood and lymphatic vessel formation. His lab has pioneered imaging techniques and genetic tools to study endothelial cell behavior and vascular patterning in vivo.