Webinars Archive
Recordings are available to members through the "members-only" portal.
Studying Hematopoiesis & Solid Tumors in Zebrafish
Presented by the Canadian Research Community
Featuring - Joey Ghersi, PhD and Madeline Hayes, PhD
January 23, 2025
2:00pm - 3:00pm EST | Find your time here

Defining the origin of blood stem cell heterogeneity
Joey Ghersi, PhD
Group Leader
CHU Sainte Justine
Assistant Professor
Department of Pathology and Cell Biology
University of Montreal
@joey_ghersi @joeyghersi.bsky.social
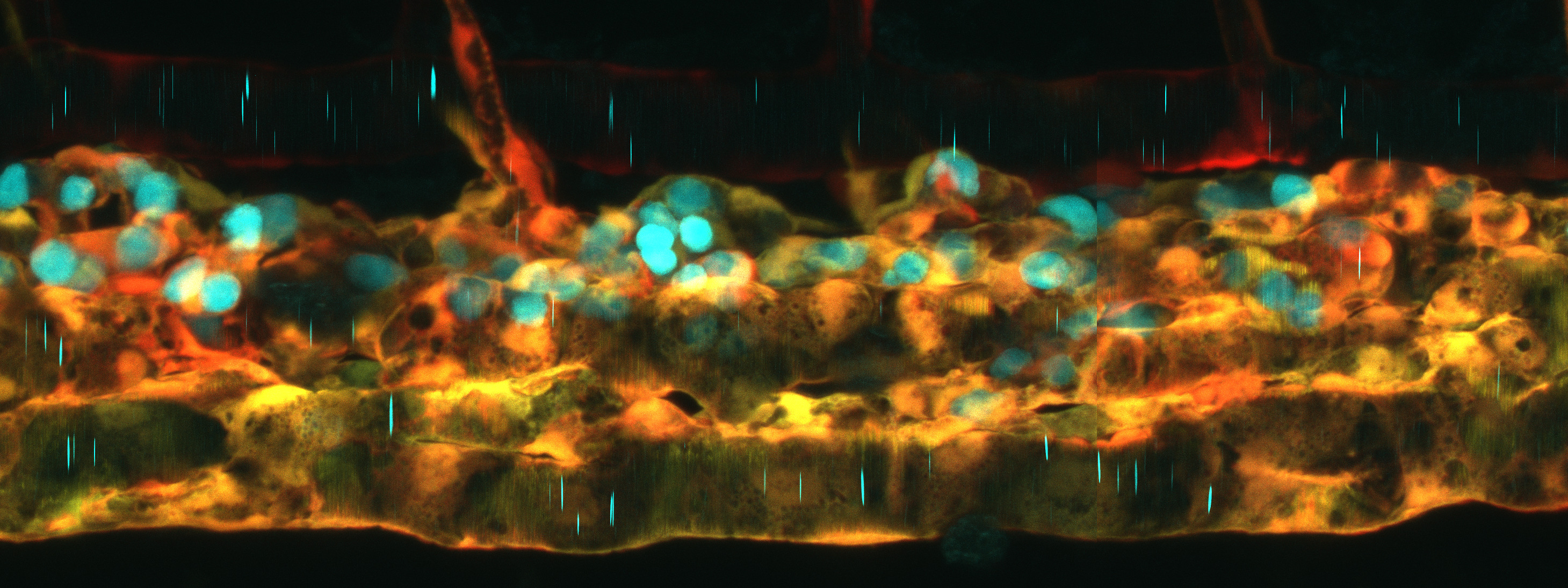
Stem cell populations across tissues, long thought to be homogeneous, have been identified as heterogeneous. Heterogeneity is defined by diverse phenotypic and functional cell characteristics. Stem cell heterogeneity must be properly regulated, or it can lead to lineage-biased progeny and a decreased capacity to repopulate tissues during regeneration. Single-cell sequencing analyses show that adult hematopoietic stem and progenitor cells (HSPCs) are a heterogeneous mixture of multipotent stem and progenitor cells that differ in cell cycle status, transcriptional lineage priming, and blood lineage outputs. Reprogramming of somatic cells to HSPCs has been the holy-grail for autologous transplantation, a lifesaving therapy against a variety of blood cancers. These cells show variations in self-renewal kinetics and lineage priming biases that can compromise the balanced reconstitution of blood and immune cells after transplantation. Diverse HSPC phenotypes are observed during development in the aorta gonad mesonephros (AGM) of the embryo, where HSPCs are first made. These data suggest that HSPCs are “born heterogeneous”. However, the basis of this intrinsic heterogeneity of HSPCs remains unknown. Uncovering this mechanism will help inform new strategies to regulate HSPC phenotypes ex-vivo and in-vivo. We discovered that signaling in endothelial cells, before the endothelial to hematopoietic transition, regulates HSPC heterogeneity in the embryo and adult zebrafish (Nat Cell Bio, 2023). We found that loss of the microRNA in endothelial cells, miR-128, alters the composition of these HSPC states. miR-128 directly inhibits the activity of Wnt– and Notch-signaling in endothelial cells. Mechanistically, single-cell RNA-sequencing revealed that Wnt regulation, instructs the transdifferentiation of replicative and erythroid-biased HSPCs. In contrast, Notch regulation programs lymphoid-biased HSPCs. These results showed that coordinated regulation of multiple signaling pathways in endothelial cells, ultimately controls HSPC heterogeneity. We propose that HSPC heterogeneity is established in the AGM endothelium prior to transdifferentiation and is programmed in part by Wnt and Notch signaling modulation.

Patient-specific in vivo modeling of high-risk extracranial pediatric solid tumors
Madeline Hayes, PhD
Group Leader
SickKids
Assistant Professor
Department of Molecular Genetics
University of Toronto
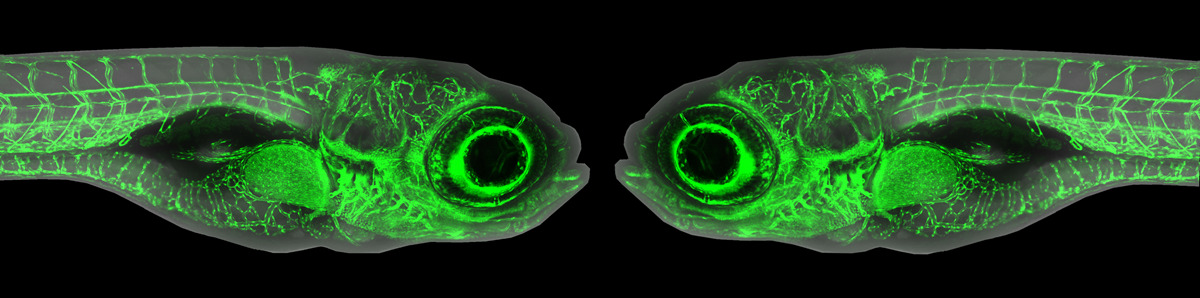
Precision oncology programs have revolutionized our ability to understand pediatric cancers on a genetic level. However, functional roles for novel variants in tumor progression and treatment response remain poorly understood. My lab focuses on using zebrafish and human cell-based models to interrogate roles for variants identified in patients with aggressive pediatric solid tumors, including neuroblastoma and rhabdomyosarcoma. Our recent findings demonstrate important roles for DNA Damage Response (DDR) genes in neuroblastoma pathogenesis and have led to the identification of novel therapeutic combinations to target these tumors. Furthermore, our models implicate less differentiated mesenchymal-like cells in solid tumor metastasis and I will discuss how we are using various in vivo tools to characterize aggressive tumor cell types, with the goal of identifying effective candidate therapies for future translation to early-phase clinical trials.
Neuroscience & Methods/CRISPR
Featuring - Ethan Scott, PhD & Saba Parvez, PhD
November 21, 2024
2:00pm - 3:00pm ET | Find your time here

MIC-Drop-seq: Large-scale in vivo mutagenesis and parallel phenotyping at cellular resolution
Saba Parvez, PhD
Assistant Professor
Cell and Developmental Biology
Feinberg School of Medicine, Northwestern University
Chicago, IL, USA
@sparvez21
A major goal of developmental biology is elucidating the relationships between genotypes and phenotypes during embryonic development. Most studies have been limited to studying a single genotype at a time, and the phenotypes measured are often narrowly focused on a specific morphological change. Moreover, phenotypes are often difficult to compare across multiple genotypes. To accelerate the mapping of genotypes to developmental phenotypes, we created MIC-Drop-seq, a method that combines high-throughput CRISPR-based gene editing (MIC-Drop) with parallel molecular phenotyping by single-cell RNA sequencing (scRNAseq). In a single MIC-Drop-seq experiment, we delivered a library of 50 CRISPR perturbations of highly expressed transcription factors to zebrafish embryos and recovered ~239,000 cells for phenotypic analysis. Cell type-specific abundance and gene expression data recapitulated many known mutant phenotypes and identified novel roles for transcription factors in brain and mesoderm development..

Brain-wide circuitry underlying altered auditory habituation in zebrafish models of autism
Ethan Scott, PhD
Professor and NHMRC Investigator
Head, Lab for Neural Circuits and Behaviour
Director of Research, Department of Anatomy and Physiology
The University of Melbourne
@LabEthan
Auditory processing is widely understood to occur differently in autism, though the patterns of brain activity underlying these differences are not well understood. The diversity of autism also means brain-wide networks may change in various ways to produce similar behavioral outputs. We used larval zebrafish to investigate auditory habituation in four genetic lines relevant to autism: fmr1, mecp2, scn1lab and cntnap2. In free-swimming behavioral tests, we found each line had a unique profile of auditory hypersensitivity and/or delayed habituation. Combining the optical transparency of larval zebrafish with genetically encoded calcium indicators and light-sheet microscopy, we then observed brain-wide activity at cellular resolution during auditory habituation. As with behavior, each line showed unique alterations in brain-wide spontaneous activity, auditory processing, and adaptation in response to repetitive acoustic stimuli. We also observed commonalities in activity across our genetic lines that indicate shared circuit changes underlying certain aspects of their behavioral phenotypes. These were predominantly in regions involved in sensory integration and sensorimotor gating rather than primary auditory areas. Overlapping phenotypes include differences in the activity and functional connectivity of the telencephalon, thalamus, dopaminergic regions, and the locus coeruleus, and excitatory/inhibitory imbalance in the cerebellum. Unique phenotypes include loss of activity in the habenula in scn1lab, increased activity in auditory regions in fmr1, and differences in network activity over time in mecp2 and cntnap2. Comparing these distinct but overlapping brain-wide auditory networks furthers our understanding of how diverse genetic factors can produce similar behavioral effects through a range of circuit- and network-scale mechanisms.
Development of Meninges (Lymphatics) & Ionocytes
Featuring - Julia Peloggia and Marina Venero Galanternik, PhD
September 19, 2024
12:00pm-1:00pm EST | Find your time here

Environmental and molecular control of tissue-specific ionocyte differentiation
Julia Peloggia
Postgraduate Researcher
Stowers Institute for Medical Research
https://twitter.com/jupeloggia
Organisms cope with environmental fluctuations and maintain fitness in part via reversible phenotypic changes (acclimation). Aquatic animals are subject to dramatic seasonal fluctuations in water salinity, which affect osmolarity of their cells and consequently cellular function. Mechanosensory lateral line hair cells detect water motion for swimming behavior and are especially susceptible to salinity changes due to their direct contact with the environment. To maintain hair cell function when salinity decreases, neuromast (Nm)-associated ionocytes differentiate and invade lateral line neuromasts. The signals that trigger the adaptive differentiation of Nm ionocytes are unknown. We demonstrate that new Nm ionocytes are rapidly specified and selectively triggered to proliferate by low Ca2+ and Na+/Cl- levels. We further show that Nm ionocyte recruitment and induction is affected by hair cell activity. Once specified, Nm ionocyte differentiation and survival are associated with sequential activation of different Notch pathway components, a process different from other tissue-specific ionocytes. In summary, we show how environmental changes activate a signaling cascade that leads to physiological adaptation. This may prove essential for survival not only in seasonal changing environments but also changing climates.

From wrappers to drains: Anatomical and Molecular Characterization of the Zebrafish Meninges
Marina Venero Galanternik, PhD
Assistant Professor
Department of Human Genetics
University of Utah
https://twitter.com/jupeloggia
Research in the Venero Galanternik lab focuses on the meninges, a set of membranous tissues that envelop and protect the vertebrate central nervous system (CNS). Meningeal dysfunction due to disease is both highly prevalent and associated with high levels of morbidity and mortality. Despite their critical roles, little is known about resident meningeal cell populations and how they work together to accomplish their important protective functions. The genetic and experimental accessibility of the zebrafish, combined with the ability to perform high-resolution optical imaging of the brain surface through the thin, transparent skull of developing and even adult animals, make zebrafish an ideal research organism for studying the meninges. Our lab has established a strong anatomical, cellular and molecular foundation for zebrafish meningeal research and our ongoing goals are focused on the understanding of the biological events regulating meningeal formation, meningeal cells functions, and investigating the potential crosstalk between the meninges and their adjacent tissues during homeostasis and disease.
Single Cell Phenotyping and Developmental Toxicology
Featuring - Subham Dasgupta, BS, MSc, PhD & Lauren Saunders, PhD
May 23, 2024
11:00am - 12:00pm ET | Find your time here

Flame retardant tetrabromobisphenol A (TBBPA) disrupts histone acetylation during zebrafish maternal-to-zygotic transition
Subham Dasgupta, BS, MSc, PhD
Assistant Professor
Department of Biological Sciences
Clemson University
https://twitter.com/devtoxx
Tetrabromobisphenol A (TBBPA) is a widely used brominated flame-retardant utilized in the production of electronic devices and plastic paints. Within this presentation, I will talk about using zebrafish as a model to study the developmental effects of TBBPA during maternal-to-zygotic transition- a pre-pluripotent embryonic stage that establishes the zygotic genome. We found that exposures to TBBPA delays development and inhibits activation of the zygotic genome and these effects are potentially caused by TBBPA-induced inhibition of histone acetylation through a histone acetyltransferase-mediated regulation.

Developmental genomics from single cells to organisms
Lauren Saunders, PhD
Junior Professor
Centre for Organismal Studies at Heidelberg University
Heidelberg, Germany
https://twitter.com/LSaund11
Auto-Immune Disease & Evo-Devo
Featuring - Tracey Ollewagen, PhD and Wei Wang, PhD
April 18, 2024
8:00-9:00am EDT | 1:00-2:00pm GMT

The use of zebrafish models in auto-immune disease-related myocarditis.
Tracey Ollewagen, PhD
Postdoctoral Fellow
Experimental Medicine Research, Dept Internal Medicine
Stellenbosch University
Rheumatic diseases are associated with considerable systemic inflammation. This results in substantial damage to various additional organs and tissues. Furthermore, this is heightened in African populations due to potential genetic differences and delayed diagnoses and treatment. This presentation will discuss how we use zebrafish to model these comorbid aspects of rheumatic disease, focusing specifically on myocarditis and the associated inflammation and fibrosis, and suggestions on future therapies.

Evolution of regeneration in teleost fish
Wei Wang, PhD
Assistant Investigator
National Institute of Biological Sciences, Beijing (NIBS)
Tsinghua Institute of Multidisciplinary Biomedical Research, Tsinghua University
@weiwang0128
Regeneration is not evenly distributed in nature. Some animals (e.g., fish and salamanders) regenerate extensively while others such as mammals regenerate poorly. The genetic mechanisms underlying the evolution of regeneration are not well understood. Using caudal fin as a model, we observed a diversity in the efficiency of fin regeneration in different teleosts. In my presentation, I will discuss the molecular and cellular mechanisms responsible for such diversity.
Evo-Devo; Enhancers and Innate Immunity
Featuring - Alexa Burger, PhD and Serge Mostowy, PhD
March 21, 2024
9:00-10:00am EDT | 1:00-2:00pm GMT

Conserved distant enhancers regulate Brachyury/T/Tbxt expression in the vertebrate notochord
Alexa Burger, PhD
Associate Research Professor
Section of Developmental Biology, Department of Pediatrics, School of Medicine
University of Colorado Anschutz Medical Campus
@aburger2009
The cell type-specific expression of key transcription factors is central to development and disease. Brachyury/T/TBXT is a major transcription factor for gastrulation, tailbud patterning, and notochord formation; however, how its expression is controlled in the mammalian notochord has remained elusive. We identified three conserved Brachyury-controlling notochord enhancers, T3, C, and I, in the mammalian Brachyury/T/TBXT locus that are active in transgenic assays in zebrafish, axolotl, and mouse. Acting as Brachyury-responsive, auto-regulatory shadow enhancers, in cis deletion of all three enhancers in mouse abolishes Brachyury/T/Tbxt expression selectively in the notochord, causing specific trunk and neural tube defects without gastrulation or tailbud defects. The three Brachyury-driving notochord enhancers are conserved beyond mammals in the brachyury/tbxtb loci of fishes, dating their origin to the last common ancestor of jawed vertebrates. Our data define the vertebrate enhancers for Brachyury/T/TBXTB notochord expression through an auto-regulatory mechanism that conveys robustness and adaptability as ancient basis for axis development.

Use of zebrafish to study Shigella-phagocyte interactions
Serge Mostowy, PhD
Professor of Celluar Microbiology
London School of Hygiene and Topical Medicine
@MostowyLab
We discovered that host cells can prevent the actin-based motility of Shigella by compartmentalizing bacteria inside septin cages for targeting to autophagy. From this we hypothesized that understanding the role of septins, an enigmatic component of the cytoskeleton, will provide insights into bacterial infection and cellular immunity. We recently pioneered a ‘bottom up’ cellular microbiology approach to study cellular immunity and discovered a fundamental link between bacterial cell biology and the assembly of septin cages around Shigella. A major issue is now to fully decipher the underlying molecular and cellular events, and to validate these events analyzed in vitro during bacterial infection in vivo using relevant animal models. We developed zebrafish as an important model to study the cell biology of infection and therapeutic potential of targeting the cytoskeleton in vivo. Our findings should be of interest for both cell and infection biologists, and provide cutting edge platforms for studies at the single cell and whole animal level.
Diabetes and ARV's & Neuroscience
Featuring - Mlondi Shezi and Summer Thyme, PhD
February 22, 2024
8:00-9:00am EST | 1:00-2:00pm GMT

Diencephalic and Neuropeptidergic Dysfunction in Zebrafish with Autism Risk Mutations
Summer Thyme, PhD
Assistant Professor
UMass Chan Medical School
@summerbthyme
Link to bioRxiv paper: https://www.biorxiv.org/content/10.1101/2024.01.18.576309v1
"Hundreds of human mutations are linked to autism and related disorders, yet the functions of many of these mutated genes during vertebrate neurodevelopment are unclear. We generated 27 zebrafish mutants with presumptive protein-truncating mutations or specific missense variants corresponding to autism-risk alleles in 17 human genes. We observed baseline and stimulus-driven behavioral changes at larval stages, as well as social behavior differences in lines tested as juveniles. Imaging whole-brain activity revealed a near identical activity map for mutations in the unrelated genes kmt5b and hdlbpa, defined by increased activity mainly in the diencephalon. Mutating 7 of the 17 risk genes resulted in substantial brain size differences. Using RNA sequencing, we further defined molecular drivers of the observed phenotypes, identifying targetable disruptions in neuropeptide signaling, neuronal maturation, and cell proliferation. This multi-modal screen nominated brain regions, cell types, and molecular pathways that may contribute to autism susceptibility."

Evaluating the Effect of Embryonic Exposure to Antiretroviral Therapy (ART) on the development of Type-2 Diabetes Mellitus (T2DM) in a Zebrafish Model
Mlondi Shezi
Ph.D. candidate (thesis on review) Biochemistry; University of KwaZulu-Natal.
Masters and honors in Biochemistry at the University of KwaZulu-Natal, South Africa.
Technician at the Zebrafish Research Unit (ZRU): University of KwaZulu-Natal.
People living with HIV (PLWH), have a high risk of developing metabolic disorders such as hyperglycemia and type 2 diabetes mellitus (T2DM) as a result of antiretroviral therapy (ART). TLD, the first-line ART regimen in South Africa is associated with adverse side effects such as hyperglycemia, bodyweight gain, T2DM; and is widely used for viral suppression in HIV-positive pregnant women. The mechanisms underlying these side effects are not well understood, especially the implications of in-utero infant exposure later in life. We, therefore, utilized zebrafish to increase our understanding of the effects of embryonic TLD exposure on the development of T2DM in adult organisms, with post-natal bodyweight, fasting blood glucose, and the expression of hormones and enzymes central to glucose metabolism as indicator endpoints.
Hematopoietic Stem Cells & Stress and Immunity
Featuring - Lili Jing, PhD and Amrutha Swaminathan, PhD
January 18, 2024
8:00-9:00am ET | 1:00-2:00pm GMT

The aryl hydrocarbon receptor (AHR) signaling regulates normal myeloid development and induces AML cell differentiation
Lili Jing, PhD
Associate Professor
Shanghai Jiao University, China
Most acute myeloid leukemias (AML) lack effective treatment. AML is characterized by arrested differentiation and uncontrolled proliferation of myeloid immature cells. Inducing AML cell differentiation is an effective therapeutic strategy. Zebrafish Tg(drl:hoxa9) drives overexpression of hoxa9 in hematopoietic cells, and exhibits strong myeloid differentiation arrest. Using Tg(drl:hoxa9), we performed a chemical screen and identified that an aryl hydrocarbon receptor (AHR) agonist potently restored myeloid differentiation. We also found that AHR signaling regulates myeloid development during embryonic development and that AHR agonists promote myeloid differentiation of various leukemic cells. We further found that AHR activation cross-talks with ATRA signaling and enhances ATRA-induced myeloid differentiation. In this webinar, we will also discuss the potential mechanism by which AHR activation regulates myeloid development and differentiation, and its potential therapeutic use in AML.

How do immune factors regulate the establishment of behaviour?
Amrutha Swaminathan, PhD
Assistant Professor
Indian Institute of Science Education and Research (IISER) Thiruvananthapuram
Immune factors are increasingly being recognized to play other non-canonical roles in additional to their known function. I will discuss our recent work where we discovered that immune factors regulate the establishment of stress-responsive behaviour in zebrafish.
“For my Ally is the Force”: Engineering Sensors for in vivo Imaging of Mechanosensation
Featuring - Periklis (Laki) Pantazis, PhD and Konstantinos Kalyviotis, PhD
Dan Gorelick, PhD, Jessica Plavicki, PhD and Ben Lovely, PhD
July 17, 2023
10:00-11:00am ET
Cellular mechanosensation and mechanotransduction are inherent abilities of cells to perceive and respond to extrinsic and intrinsic mechanical stimuli. In this webinar, we will discuss engineering efforts aimed at developing biosensors for mechanical interrogation of biological systems. We will guide everyone through the insights gained from our research in developing a Piezo1 activity sensor called GenEPi (Genetically Encoded Piezo1 indicator), and we will also suggest future engineering approaches to visualize the unseen forces within cells.
 |
 |
|
Dr Konstantinos Kalyviotis |
Dr Periklis (Laki) Pantazis |
The ins (absorption) and outs (excretion) of zebrafish toxicology studies
Featuring - Dan Gorelick, PhD, Jessica Plavicki, PhD and Ben Lovely, PhD
April 19, 2023
10:00-11:00am ET
Zebrafish have long been a powerful model with which to study impact of environmental factors to development and disease. In this webinar, we will discuss approaches, controls and potential hurdles of environmental studies by discussing tips and tricks we have learned and developed in our research programs. Our goal is to provide an open dialogue of the “ins (absorption)” and “outs (excretion),” of toxicology/teratology studies.
 |
 |
 |
| Dr. Dan Gorelick Assistant Professor Baylor College of Medicine |
Dr. Jessica Plavicki Assistant Professor Brown University |
Dr. Ben Lovely Assistant Professor University of Louisville |
The zebrafish epigenome: past, present, and beyond
Featuring - Ozren Bogdanovic, PhD & Ferenc Mueller, PhD
February 9, 2023
9:00-10:00am ET
Dr. Ozren Bogdanovic will discuss: “Epigenome reprograming during zebrafish embryogenesis”
Dr. Ferenc Mueller will discuss: “How to exploit DANIO-CODE resources for gene regulation”
Vertebrate embryogenesis requires tight orchestration of spatiotemporal gene expression patterns, which is achieved through the coordinated action of transcription factors and genomic regulatory marks such as DNA methylation and histone tail modifications. The zebrafish model system has been instrumental for our understanding of how these marks participate in vertebrate embryogenesis. Systematic functional annotation of the zebrafish genome achieved by the DANIO-CODE consortium and by other functional annotation efforts led to creation of a central repository to store and process zebrafish developmental functional genomic data. These resources aid streamlined access to diverse zebrafish genomics and epigenomics datasets. The zebrafish user community is encouraged to exploit and to contribute to these resources.
 |
 |
| Dr. Ozren Bogdanovic Principal Investigator CABD |
Dr. Ferenc Mueller Professor University of Birmingham |
Practical considerations and approaches to zebrafish transgenesis:
Featuring - Christian Mosimann, PhD and Kristen Kwan, PhD
May 24, 2022
11:00am-12:00pm ET
Trangenesis to generate reporters, effectors, and modifier lines in zebrafish has become a standard method in the field. In this webinar, we will discuss practical considerations of transgene design, involved components, steps for quality control, and more. We will solicit questions from the community to address common problems with transgenesis.
 |
 |
|
Kristen Kwan, PhD, |
Christian Mosimann, PhD, |
Live Imaging and multi-color labelling strategies for tracking cells and circuits over time
Featuring - Fabienne Poulain, PhD and Elke Ober, PhD
May 3, 2022
10:00-11:00am ET
Dr. Fabienne Poulain will discuss “It gets better with time: dynamic refinement of neural circuits in vivo”
Dr. Elke Ober will discuss "FRaeppli, a multispectral imaging toolbox for cell tracing and dense tissue analysis"
Drs. Fabienne Poulain and Elke Ober will present recently developed transgenic approaches to label and visualize individual cells or specific cell populations during organ and circuit formation and maturation in real time. Their studies focus on deciphering processes in dense tissues at high spatial and temporal resolution.
 |
 |
| Dr. Fabienne Poulain, Assistant Professor Dept of Biological Sciences University of South Carolina |
Dr. Elke Ober, Associate Professor NNF Center for Stem Cell Biology (DanStem) University of Copenhagen, Denmark |
Optogenetics: Approaches in Zebrafish
Featuring - Stephanie Woo, PhD and Clare Buckley, PhD
April 20, 2022
11:00am-12:00pm ET
Stephanie Woo, Ph.D. will discuss "Optimizing a light-activated gene expression system for use in zebrafish"
Dr. Clare Buckley will discuss “Using optogenetics in zebrafish to understand neural tube development”
Optogenetics is a powerful emerging tool for studying biological processes, allowing high spatiotemporal control over gene expression and protein signalling. In this webinar, we will discuss the development of TAEL/C120, a light-activated inducible expression system that is specifically optimized for use in zebrafish. We will also discuss optimisation of Phytochrome optogenetics in zebrafish, which can be used to manipulate protein localisation and signalling. We will demonstrate different applications of these systems, methods for light delivery and other practical considerations.
 |
 |
| Stephanie Woo, PhD University of California Merced Merced, California |
Clare Buckley, PhD University of Cambridge Cambridge, London, UK |
Fish, Water and Sustainability: Looking to the Future
March 17, 2022
10:00 - 11:00 a.m. EDT
As researchers using fish as a model system we are absolutely dependent on the amount and quality of the water we use, yet the world is facing a water crisis characterized by both water scarcity and pollution. In this webinar I will cover how we incorporate fish, water, and sustainability into our science outreach program for people of all ages. I will also discuss water use in zebrafish facilities highlighting the magnitude of inefficient water use, potential solutions, and a project we are initiating in our fish facility to recover water. https://cienciaaltiro.cl/en/proyects/water-the-root-of-life/

Kate Whitlock, PhD
Universidad de Valparaiso, Valparaiso, Chile
Generation and Application of Conditional Knock-In Alleles in Zebrafish
September 28, 2021
10:00 - 11:00 a.m. EDT
Despite the advent of genome editing approaches in zebrafish, eliminating gene function in a cell- and stage-specific remains challenging. In this webinar, I will present our efforts to implement the Cre/lox system in zebrafish for this purpose. I will describe optimized conditions for single step generation of lox-flanked knock-in alleles. I will also provide a framework for application of zebrafish conditional knock-in alleles with inducible Cre driver lines to investigate cell- and stage-specific genetic function, including common pitfalls and essential steps for technical validation.

Nathan Lawson, PhD
University of Massachusetts Medical School
New Developments in Vascular Biology
July 1, 2021
10:00 - 11:00 a.m. ET
Please join us for a pair of dynamic presentations highlighting innovative advances in our understanding of vascular biology in zebrafish.
Dr. Karina Yaniv will discuss "The Role of Endothelial Cell "Biography" on Vascular Diversity"
Dr. Arndt Siekmann will discuss "The Making and Shaping of Blood Vessels: Zebrafish Fins are Taking the Center Stage"
|
Karina Yaniv, PhD |
Arndt Siekmann, PhD |
Innovations in zebrafish chromatin analysis: CUT&RUN, CUT&TAG and ChIP methods
May 4, 2021
9:00 - 10:00 a.m. ET and
4:00 - 5:00 p.m. ET
Zebrafish have emerged as a powerful model for understanding chromatin changes in the context of embryonic development. This seminar will highlight recent advances in understanding chromatin regulation during early embryogenesis, and will discuss how new technologies of CUT&RUN and CUT&TAG can facilitate chromatin analysis.
|
Mary Goll, PhD |
Patrick Murphy, PhD |
Disease Modeling: Discovery and Modeling of Undiagnosed Human Diseases
April 6, 2021
11:00 am - 12:00 p.m. ET and
8:00 - 9:00 p.m. ET
There are more than 400 million people worldwide who suffer from undiagnosed diseases. Many of these diseases are extremely rare with as few as a single affected individual. Zebrafish play a central role in validation of unknown disease genes and elucidation of the underlying mechanisms of pathphysiology. In this webinar, I will discuss our work as part of the Undiagnosed Diseases Network.

Monte Westerfield, PhD
University of Oregon
Neuro-Journey: From Forming Neuronal Connectivity to Generating Behavior
March 25, 2021
11:00 a.m. - 12:00 p.m. ET
Join us for an exciting webinar featuring a pair of dispatches on cutting-edge neuroscience research in zebrafish, spanning topics from the establishment of neuronal connectivity to the execution of lateralized motor behavior!
|
Harold Burgess, PhD |
Corinne Houart, PhD |
Paper Writing
February 17, 2021
12:00 - 1:00 p.m. ET

Katherine Brown, PhD
Development

Cecilia Moens, PhD
Fred Hutchinson Cancer Research Center

Didier Stainier, PhD
Max Planck Institute
In this webinar, we plan to cover various aspects of manuscript submission and publication – from journal selection, manuscript and cover letter preparation and communications with the editor, through to revisions, rebuttals and finalisation of the article. We hope this will be an interactive session and encourage participants to submit questions in advance and contribute to the discussion.
Evolutionary Studies:
Just Under the Surface: Leveraging Zebrafish to Understand the Interplay Between Evolution and Development
January 14, 2021
9:00 - 10:00 a.m. ET
6:00 - 7:00 p.m. ET

Matthew Harris, PhD
Harvard Medical School
Not only is zebrafish a powerful model to understand gene function in development, this fish provides a valuable window to understand how evolution has shaped development, and conversely, how development shapes evolution. This talk will discuss how genetic analysis in this singular representative species can support broader analysis of all fishes, including us.
Drug Discovery: Can Zebrafish Screens Really Lead to New Human Medicines?
December 10, 2020
10:00 - 11:00 a.m. ET
7:00 - 8:00 p.m. ET

Randy Peterson, PhD
University of Utah
As the only vertebrate model organism amenable to scalable screens, the zebrafish has long been touted as having the potential to deliver new drug candidates. In the past few years, drug candidates discovered in zebrafish have finally begun to reach human trials, suggesting that the potential may be turning to reality. This webinar will illustrate the arc of zebrafish drug discovery from academic lab to the clinic as well as highlight key challenges and emerging opportunities in zebrafish drug discovery.
Cancer Modeling
November 17, 2020
9:00 - 10:00 a.m. ET
3:00 - 4:00 p.m. ET

Richard White, MD, PhD
Memorial Sloan Kettering Cancer Center
In this webinar, we will discuss how the intersection of developmental programs and the microenvironment drive melanoma progression and metastasis. Additionally, we will touch on new transgenic technologies in zebrafish, including Transgene Electroporation (TEAZ) and combinatorial methods.
Imaging Workshop
October 20, 2020
12:00 - 2:00 p.m. ET
| Speaker | Talk Title |
| Jan Huisken, PhD - Morgridge Institute for Research | Zebrafish Light Sheet Imaging With a Modular, Portable Microscope |
| Elizabeth Hillman, PhD - Columbia University | Broad Applications of SCAPE Microscopy for High-Speed 3D Imaging in Zebrafish Larvae |
| Misha Ahrens, PhD - HHMI Janelia Research Campus | Imaging Neuronal and Glial Activity in Behaving Zebrafish |
| Kristen Kwan, PhD - The University of Utah | Computational Approaches to Dissecting Developmental Tissue Morphogenesis |
| Elisabeth Kugler, PhD - University College London | Quantifying the 3D Zebrafish Brain Vasculature in LSFM Data |
| Jonas Hartmann, PhD - University College London | Exploring Morphogenesis with Imaging and Data Science |
Moderator: Debbie Yelon, PhD - University of California San Diego
Imaging and image analysis are central components of many types of zebrafish research. The presentations in our imaging workshop will highlight a variety of innovative approaches for capturing biologically informative images and for performing insightful image analysis.
Single Cell Genomics
September 29, 2020
12:00 - 1:00 p.m. ET

Tatjana Sauka-Spengler, PhD
University of Oxford
How to Interview for a Zebrafish PI Position Workshop
August 27, 2020
9:00 - 10:30 a.m. ET


Phil Ingham, PhD Lila Solnica-Krezel, PhD
Phil Ingham, PhD - Nanyang Technological University
Bushra Raj, PhD - Harvard University
Lila Solnica-Krezel, PhD - Washington University School of Medicine
Nadine Vastenhouw, PhD - Max Planck Institute
Participate in this workshop to learn how to best prepare for an interview and gain insight about the interviewing process for a Zebrafish PI position. This webinar will feature an interactive Q&A session with these four leaders.
Online Genome Editing Workshop
July 22, 2020
9:00 - 11:00 a.m. ET
While generating mutations at specific loci has become routine, more sophisticated forms of genome editing in zebrafish remain challenging. Four leaders in this field will be sharing their latest data and advice.
David Grunwald, PhD - University of Utah
Bo Zhang, PhD - Peking University
Filippo Del Bene, PhD - Institut de La Vision
Darius Balciunas, PhD - Temple University
Moderator: Didier Stainier, PhD - Max Planck
Zebrafish Husbandry - Environmental and Husbandry Limits on the Growth of the Zebrafish Model System: What We Need to Improve Upon and How We Do It
June 23, 2020 at 11:00 a.m. and 3:00 p.m. ET

Christian Lawrence
Join expert, Christian Lawrence, in an one-hour webinar that will center around key aspects of zebrafish environmental management and husbandry that are critical to the continued growth and applicability of the model system. Recent scientific and technological advances in nutrition, welfare, population genetics, sanitization, and pathogen control will be highlighted, and their implications for the field will be discussed. An interactive Q&A session will follow the presentation.
Christian Lawrence directs the Aquatic Resources Program (ARP) at Boston Children’s Hospital (BCH). ARP administers the zebrafish program at BCH, which is one of the largest and most active of its kind in the world. Mr. Lawrence also serves as a faculty member for the Health and Colony Management of Laboratory Fish course at the Mount Desert Island Biological Laboratory, and was a Fulbright Specialist at the Bar-Ilan University Faculty of Medicine in Safed, Israel in 2013. He is co-author of The Laboratory Zebrafish, and has written a number of scientific publications on zebrafish biology and culture.
Zebrafish Cardiac Regeneration Workshop
June 15, 2020
10:00 a.m. - 12:30 p.m. ET
Zebrafish have become a very popular model to investigate mechanisms of cardiac regeneration following the pioneering studies by Ken Poss, PhD, in 2002. This webinar will feature presentations by six leaders in this very active field followed by a round table discussion.
Ken Poss, PhD - Duke University Medical Center
Caroline Burns, PhD - Boston Children's Hospital
Kazu Kikuchi, PhD - National Cerebral and Cardiovascular Center
Gilbert Weidinger, PhD - Ulm University
Jeroen Bakkers, PhD - Hubrecht Institute
Nadia Mercader, PhD - University of Bern
Moderator: Didier Stainier, PhD - Max Planck
Grant Writing Basics
May 28, 2020 at 11:00 a.m. and 9:00 p.m. ET

Mary Mullins, PhD
Writing a strong grant proposal requires compelling science, a well written narrative that emphasizes the significance and novelty of your research, and convincing evidence of your ability to accomplish the goals. Get tips on writing a strong, persuasive grant proposal, and avoiding certain pitfalls. Attend this one-hour webinar featuring Mary Mullins, PhD from the University of Pennsylvania. An interactive Q&A session will follow the presentation.
Use of CRISPR/Cas9 for Targeted Integration in Zebrafish
April 29, 2020 at 5:00 p.m. and 9:00 p.m. ET

Jeffrey Essner, PhD
Jeffrey Essner, PhD, from Iowa State University, will present the latest updates on the use of CRISPR/Cas9 for targeted integration in zebrafish.
Dr. Jeffrey Essner is a professor and zebrafish PI at Iowa State University. Over the past several years, he has collaborated with Drs. Maura McGrail, Drena Dobbs, Karl Clark and Stephen Ekker on an NIH R24 project to develop tools for the zebrafish community for targeted integration tools and conditional alleles. His talk will focus on these efforts and highlight the resources developed. A question and answer session will follow the presentation.