IZFC Meeting Report
The 16th International Zebrafish Conference, originally planned for Montreal, was held virtually on June 16-22. The silver lining of the virtual format was that the meeting brought together 950 registrants from 42 countries, with zero carbon emissions. There were three concurrent sessions and six plenary sessions on topics as diverse as the Serengeti wildlife that we learned about in Sean Carroll’s keynote lecture. My own PhD supervisor Janet Rossant gave a keynote lecture on current progress toward understanding human embryo development. There were also award presentations for both 2020 and 2021 due to the cancellation of last year’s European Zebrafish meeting.
“Beautiful Imaging!” was a frequent exclamation after many of the presentations at the meeting. This is nothing new for our field—live imaging being where the zebrafish traditionally (and literally) shines—however the meeting was notable in the array of new tools for live imaging it introduced and the inventive uses that they were put to. The meeting saw a flourishing of single-cell (or single nucleus) RNA-Seq and CRISPR-mediated insertion into endogenous genes to discover new cell types and new biology in both the embryo and the adult, in normal and mutant animals, and in health and disease.
Several talks described the identification of new cell types using scRNA-Seq: Marina Galanternik (Weinstein lab, NICHD) used it to identify novel cell types in the meninges and dura (the thin tissue layers between the brain and the skull) and then to visualize their interactions through the transparent skull of adult casper mutants – unthinkable in any other vertebrate. Vishnu Saraswathy (Mokalled lab, Wash U.) used scRNA-Seq to identify a population of neural cells that turn on myostatin—the well-known a repressor of muscle differentiation—in response to spinal cord injury and showed that in this context myostatin prevents premature neuronal differentiation and thereby promotes regeneration. scRNA-Seq helped Kenny Mattonet (Stainier lab, MPI Bad Nauheim) to make the wholly unexpected discovery that hemato-vascular progenitors transfate to pronephros in npas4l (cloche) mutants. Peter Fabian (Crump lab, USC) used scRNA-Seq of cranial neural crest-derived cells to identify an unexpected population of dermal fibroblasts that function to metabolize phenylalanine and tyrosine, thus preventing the inappropriate absorption of pigment into underlying bones. Finally, Hung-Jhen Chen (also from the Crump lab) used scRNA-Seq to identify a population of nr5a2-expressing neural-crest-derived cells that give rise to a specific tendon at a muscle insertion point on the lower jaw. In the absence of nr5a2, these cells trans-fate into lower jaw cartilage, indicating a role for nr5a2 in preventing premature differentiation of neural crest-derived cells. Remarkably, mouse nr5a2 conditional knockouts have abnormalities in the homologous bones and tendons in the middle ear.
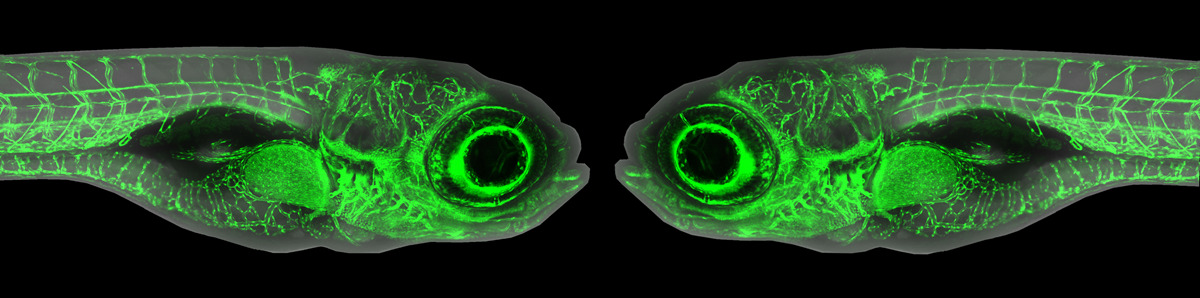
The meeting saw widespread adoption of targeted insertion of reporters into endogenous genes via homologous recombination, homology-directed repair, or homology-independent targeting. Daniel Levic (Bagnat Lab, Duke U.) recommended targeting non-coding sequences (introns or 5’UTR) when making fusion proteins by homology-directed repair to avoid generating indels that disrupt protein function, and he used the approach to make exquisite endogenous fusions for apical membrane, basolateral membrane and intracellular vesicles. Maria Jussila (Ciruna lab, Sick Kids, Toronto) introduced sfYFP into endogenous Vangl2 by homologous recombination to visualize its unique planar polarized localization, while Rohit Harish (Brand lab, TU Dresden) introduced GFP into endogenous Fgf8 to visualize its classical morphogenic activity.
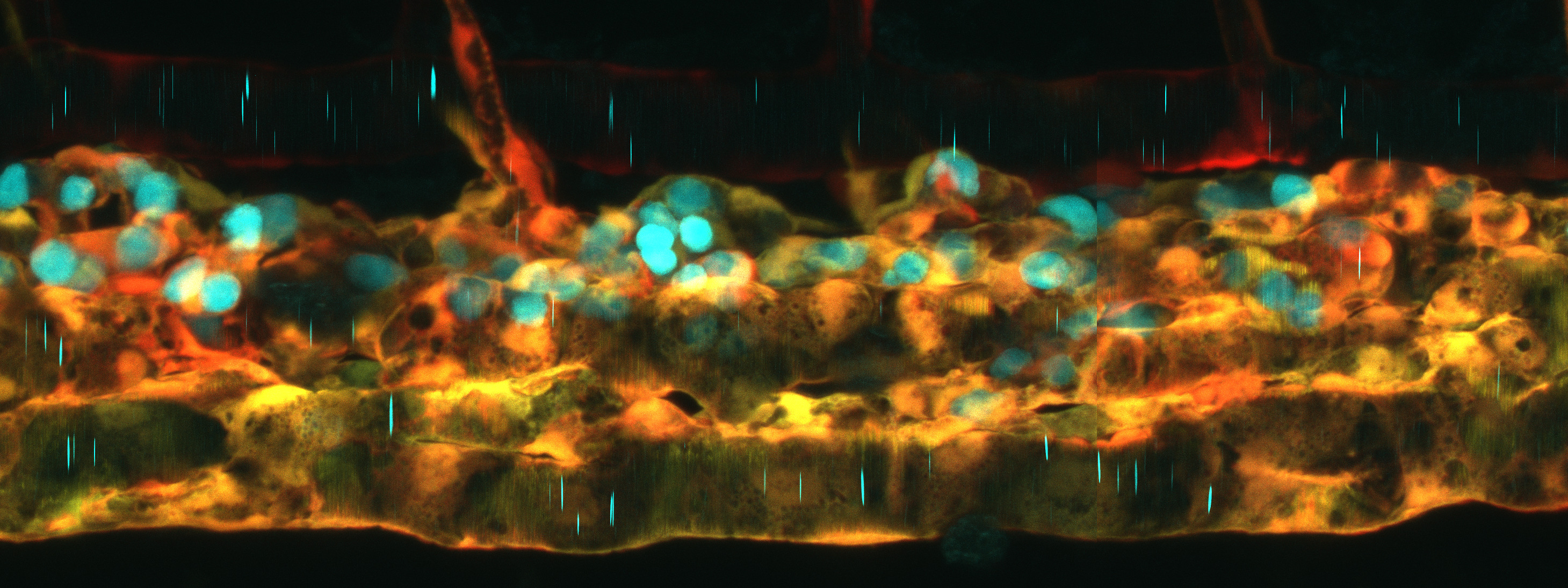
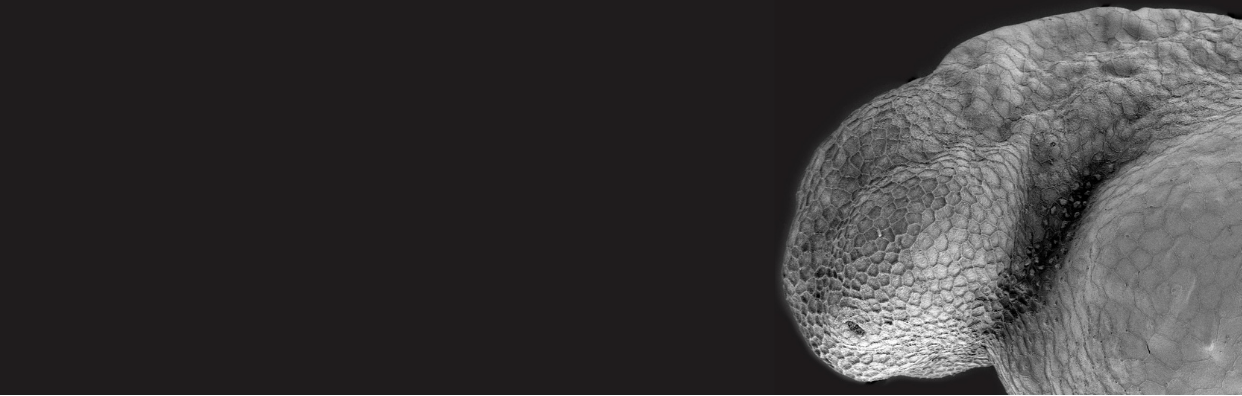
In vivo live imaging revealed exciting new cell biology in a range of contexts. Imaging the budding of the semi-circular canals of the inner ear, Akankshi Munjal[1] (Megason lab, Harvard) discovered actomyosin-based “cytocinches” that extend and retract in the circumferential axis of the bud to resist the hydrostatic pressure generated when hyaluronic acid in the lumen swells up, thereby shaping what would otherwise be an isometric bulge into an anisometrically extending tube. Elizabeth Chen (UTSW) described an actin-rich “fusogenic synapse” required for the fusion of muscle cells to make multinucleate myotubes. Imaging the development of the hepato-biliary network, Sara Caviglia (Ober lab, U. Copenhagen) described stable bleb-like structures on hepatocytes ,“BLiSTRs”, that drill into the lumen of the biliary cell to initiate the formation of the connecting canaliculus. Finally, by long-term two-photon imaging of melanoma tumors in anesthetized fish, Aya Ludin (Zon lab) discovered dynamic “craters” in the tumor that are plugged with collagen and appear to harbor CD8+ T-cells. CD8+ T-cell-mediated killing of tumor cells is a key aspect of cancer immunotherapy, so understanding and improving their access to solid tumors is of high clinical importance.
The optical qualities of the zebrafish mean that light can be used not only to visualize individual cells and tissues in living animals but also to assess their responsiveness to environmental cues or to manipulate those responses using optogenetic tools. In one exquisite example, Alessio Paolini (Abdelilah-Seyfried lab, U. Potsdam) used reporters of wnt and Notch signaling in cells of the forming atrio-ventricular canal to define the signaling environment necessary for the single-cell movements that initiate valvulogenesis. Other talks used optogenetic tools to manipulate signaling pathways to understand the principles by which they control development. Katherine Rogers (Müller lab, MPI Tübingen) used opto-BMP to reveal the importance of combinatorial signaling by BMP, FGF and Nodal to pattern the dorso-ventral axis of the early embryo[2], and in her Chi-Bin Chien lecture Margot Williams (Baylor College, Texas) used opto-Nodal to demonstrate an autonomous function for Nodal signaling in neuroepithelial convergent extension movements, parallel to known mesodermal- and planar polarity-dependent mechanisms. Finally, Luis Silva (Ninov lab, Center for Regenerative Therapies, Dresden) and Emma Spikol (Halpern lab, Dartmouth) both used targeted stimulation of Channel Rhodopsin to excite single cells of the pancreatic islet (Silva) to assess their ability to initiate Ca2+ waves that drive pulsatile insulin secretion, and to assess functional neuronal connectivity between the dorsal habenula and a novel population of gsc2-expressing neurons in the nucleus incertus (Spikol).
This short summary, with its focus on live imaging, doesn’t begin to do justice to the breadth of topics that made up this diverse and exciting meeting. Progress on a range of disease models, from autism (Hoffman lab, Yale; Patten lab, INRS Quebec[3]) to addiction (Peterson lab, U. Utah[4]) to SARS-CoV-2 anosmia (Salinas lab, U New Mexico[5]) was presented, often using ingenious behavioral paradigms. Mathematical modeling gave new insights into developmental mechanisms from directional cell migration in the gastrula (Pauli lab, IMP Austria; Heisenberg lab, IST Austria) to adult pigment pattern formation (Kelsh lab, U. Bath UK). Exciting new genetic approaches including Prime Editing (Yeh lab, Harvard MS[6]) and an ambitious CRISPR-based forward genetic screening strategy (Peterson lab, U. Utah) were presented in a Technology plenary session on the final day of the meeting. Last but not least, a fascinating session on Evolution explored fundamental questions like the origins of paired appendages (Carney lab, Lee Kong Chian School of Medicine, Singapore) and of vertebral segmentation (Harris lab, Harvard[7]).
The 2022 meeting is scheduled to be in Montreal, in-person or a hybrid of in-person and virtual. It will be organized by our new IZFC president, Lila Solnica-Krezel. I look forward to seeing many of you there!
The virtual talks and e-poster hall will be available for all IZFC registrants to view until mid-September.