Science Spotlight: Microglia phagocytose myelin sheaths to modify developmental myelination
Starting in 2020, the newsletter will highlight one or more recent papers that are of broad interest to the zebrafish community, either because they make an impactful new discovery or introduce a valuable new tool or technology. The IZFS board would like to encourage trainees who are interested in science communication to get involved in interviewing authors and writing pieces for the “Science Spotlight” section in future issues of the News Splash. If you would like to participate in preparing a piece for the next newsletter, please contact Cecilia Moens (cmoens@fredhutch.org). This month’s highlight is of Alexandria N. Hughes and Bruce Appel (2020) “Microglia phagocytose myelin sheaths to modify developmental myelination”. Nature Neuroscience PMID:32632287. We caught up with Alex and Bruce in their homes in Denver to talk to them about the work.

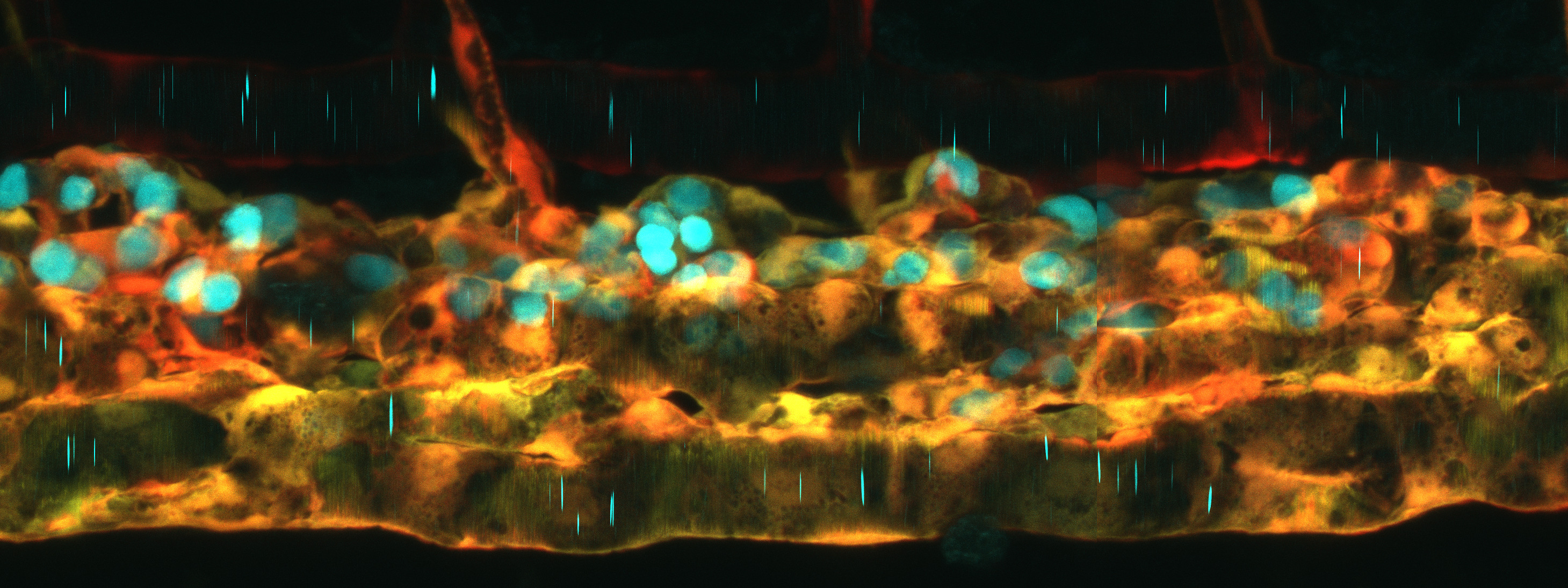
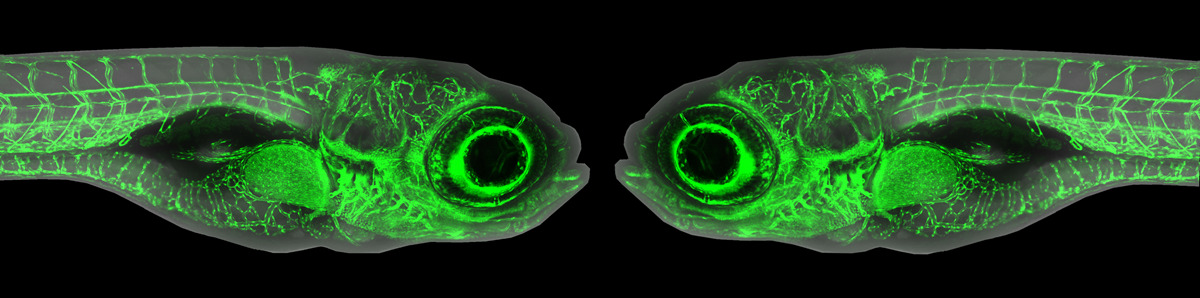
The developing nervous system is a dynamic place. Synapses form, and some are strengthened while others are eliminated – a process that depends on neuronal activity and the busy work of brain-resident macrophages called microglia that phagocytose and degrade excess synapses. Recent work has shown that myelin, the lipid-rich membrane that ensheaths axons in the central nervous system, is equally dynamic. Like synapses, myelin sheaths are produced in excess and then pared back, however the underlying mechanism was not known. In their recent paper in Nature Neuroscience (PMID:32632287), Alexandria Hughes and Bruce Appel at the University of Colorado School of Medicine discovered that microglia are also responsible for pruning myelin, and that neuronal activity regulates this process by luring microglia away from myelin.
Hughes and Appel performed live imaging of microglia, oligodendrocytes (the myelin-producing cells in the central nervous system) and nearby neurons under a range of pharmacological, genetic and chemogenetic conditions that altered neuronal activity or eliminated microglia. They discovered that microglia survey and phagocytose myelin sheaths during developmental myelination. Asked about her “Eureka! moments”, Hughes says: “The very first time I performed time lapse imaging I saw a microglial cell engulfing a myelin sheath.” However, these events were difficult to catch because fluorescent myelin fragments are so quickly broken down. Treating embryos with bafilomycin, which inhibits lysosome acidification, changed everything: “When I imaged those fish, I saw these big gobs of myelin within all of the microglia…I was able to clearly visualize that these cells were just rampaging through the spinal cord, eating myelin.” After that, Hughes’s challenge was how to quantify her imaging data. Armed with a background in mathematics and access to the biological imaging community’s many publicly available quantitative analysis tools, she was able to automate the measurement not only of engulfed myelin within microglia, but of calcium fluxes in microglia, myelin sheath morphology, and myelin sheath disappearance over time. Hughes discovered that in the tectum, microglia appear to toggle between contacting active neuronal somas and phagocytosing myelin from nearby axons. When microglia aren’t available to fulfill this sheath-pruning role, oligodendrocytes maintain extra and ectopic myelin.
“Our work answers a developmental question – how is myelin refined? – but we are also excited about the questions it opens: What is the identity of the cues or signaling molecules that specify sheaths for elimination? And how are only a subset of nascent myelin sheaths marked for phagocytosis? Finally, is myelin sheath phagocytosis important for neural circuit function? Investigating the relationship between brain activity, microglial myelin phagocytosis, and behavior should begin to uncover answers to these questions.”
About the authors: Alex Hughes is a graduate student in the Neuroscience Graduate Program at the University of Colorado. She recently defended her Ph.D. and will be moving to the NIH in 2021 to do a post-doc with Harry Burgess. She is a runner who has made COVID better by doing more reading and cooking at home. She is also a painter whose zebrafish-inspired art is available on Etsy. Bruce Appel is a Professor of Pediatrics and the head of the Section of Developmental Biology at the University of Colorado Anschutz Medical Campus since 2008. Before that he was a Professor at Vanderbilt University. He did his post-doc with Judith Eisen at the University of Oregon in the mid 1990s. His lab studies the specification, development, regulation and function of myelinating cells in the zebrafish. Outside of the lab he is an avid cyclist, hiker, backpacker, skier and snowshoer in the mountains around Denver. Asked about how he and his lab have dealt with the COVID situation, Bruce cites an increased focus on bioinformatics: trainees have been able to take time to learn how to analyze single-cell sequencing datasets and develop image analysis tools. Others have focused on writing papers and theses. Bruce himself has been in grant-writing mode. In terms of doing experiments in a lab that is capped at 50% capacity due to COVID, Bruce says “People now go in and just do what they need to do and then leave. They are being pretty productive because we have to think a little harder and plan a little better.”